A special initiative of ICAN, International Cancer Advocacy Network
Improving Survival and Quality of Life, One Patient at a Time
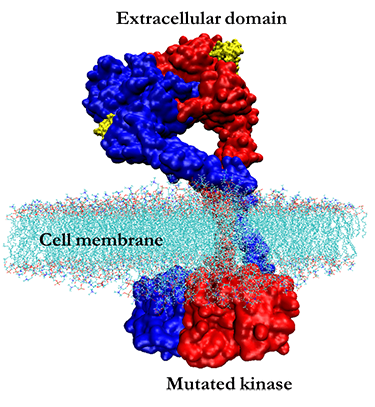
HERNEXEOS Approved for First-Line HER2 Lung/HER2 Exon 20 Insertion Mutation Therapy!
To discuss approved EGFR exon 20 drugs RYBREVANT (amivantamab), DATROWAY (datopotamab deruxtecan), access to ZEGFROVY (sunvozertinib)
and approved HER2 exon 20 drugs ENHERTU (trastuzumab deruxtecan) and HERNEXEOS (zongertinib)
and to access our Customized Clinical Trials Matching and Oncology Nursing Services, sign up to join Exon 20 Group. Membership Intake Form
Home of the EGFR Exon 20 Warriors and the HER2 Warriors!
Sign Up and Join Our Wonderful Family of Patients, Care Partners, Oncologists, and Scientists. New Patient Form
The Exon 20 Group is a laser-focused, multi-stakeholder, global coalition of patients, care partners, family members, thoracic/medical oncologists, scientists, molecular profiling labs, and pharmaceutical companies and biotechs with the mission of converting exon 20 insertion mutations into chronic diseases and then curing them. Join us!
Membership in the Exon 20 Group is FREE, but we do "onboarding" of each patient first before you can join our community. Please complete our New Patient Form. Alternatively, you may call to schedule an onboarding appointment so that you can access our numerous free services, 602-618-0183. Introductory emails describing your particular situation are welcome before we schedule our phone call with you. Send to exon20@exon20group.org
Essential Patient Services
- Ongoing, Direct Navigation of Your Case
- Review of Your Biomarker Testing Reports
- Second Opinion Referrals
- Matching You with Clinical Trials / Expanded Access / Compassionate Use Matching
- Your Own "Angel Buddy" to Help with Side Effects, and...
- The Exon 20 Group's Guide to Collateral Medications for Patients on Targeted Therapies, Elena Siltanen, MSc.Pharm
Know Your Insertion Mutation
Exon 20 Group Research Initiatives
- Invitation to our On-Demand Summit/Video Project for Patients, Scientists, and Clinicians
- Updates on our Exon 20 International Research Consortium
- Updates on Exon 20 Group-Funded Research
- Updates on our Knowledge Bank Project led by top thoracic oncologists
- Real-World Evidence Studies and Survey Research (you are compensated for your time)
- Participate in our Clinical Trials Project
The Exon 20 Community: Keeping Up With Developments
- Access to our
Exon 20 Warriors Support group for patients with EGFR Exon 20 insertions/HER2 Exon 20 insertions in lung cancer and other solid tumors . (Direct link to the Exon 20 Warriors group page)
- Access to our 19 Social Media Sites (closed group to protect your privacy)
- Access to our Virtual Patient and Care Partner Meet Ups (closed group to protect your privacy)
- Patient and Care Partner Meet-Ups at Conferences and Academic Medical Centers
- Patient and Clinician Webinars on New Drugs
- Research and Literature Updates and Daily Alerts/Postings
- Cancer Conference Updates
Paying It Forward: Helping Our Exon 20 Community
- Become an Angel Buddy for a New Patient or Care Partner
- Participate in our Public Policy Advocacy Initiatives and Briefings
- Train to be an Effective Citizen Lobbyist or Media Interviewee
- Participate in our Speakers' Bureau
- Assist our International Health Equity Efforts
- Volunteer for Clerical and Excel Projects
- Join our Research Teams
Keep the Exon 20 Group Going
- Help the Exon 20 Group Raise Funds to Keep Our Patient and Research Services Entirely Free
- Create a Facebook, Twitter, or Instagram Fundraiser Benefiting the Exon 20 Group
- Donate to Fund These Services
- Donate Appreciated Stock
- Leave a Legacy—Create a Named Program
- Donate In-Kind Services
- Support our Special Events
Membership
All you need to do to join the Exon 20 Group is:
- Please fill out the Exon 20 Group Intake Form and submit it to us.
- After reviewing your Exon 20 Group Intake Form, we will then schedule an intake phone interview with you, or with you and your family, to discuss your case and confirm your specific exon 20 insertion mutation (making sure you that you have had NGS or Next-Generation Sequencing to identify the insertion mutation).
- Alternatively, you may email exon20@exon20group.org and tell us that you are interested, and we'll schedule an Intake phone call with you or with you and your family since the exon 20 journey is very much a family affair.
Since ICAN and the Exon 20 Group strictly adhere to all HIPAA (Health Insurance Portability and Accountability Act of 1996) provisions to protect your privacy, during our initial intake call/meeting with you, we will review, and you will have a chance to discuss with us our Navigation Consent forms.
We never sell your information and only release information with your explicit permission (for example, when setting up a second opinionat your request, applying for expanded access/compassionate use, or inquiring about a clinical trial).
To volunteer to work on one of the Exon 20 Group's many satellites, task forces, or committees, please email marcia@exon20group.org
Recruiting new members for:
- Exon 20 Group-Australia/New Zealand
- Exon 20 Group-Canada
- Exon 20 Group-China
- Exon 20 Group-France
- Exon 20 Group-Germany
- Exon 20 Group-India
- Exon 20 Group-Israel
- Exon 20 Group-Italy
- Exon 20 Group-Japan
- Exon 20 Group-Jordan
- Exon 20 Group-Latin America
- Exon 20 Group-The Netherlands
- Exon 20 Group-Nordic Region
- Exon 20 Group-South Africa
- Exon 20 Group-Spain
- Exon 20 Group-United Kingdom
Exon 20 Group Leadership
click for full list and biographies
*Marcia K. Horn is President and CEO of
ICAN International Cancer Advocacy Network and
Executive Director of
the Exon 20 Group.
The Exon 20 Group is a special initiative of